The pro-cognitive mechanisms of physical exercise in people with schizophrenia
Abstract
Schizophrenia is associated with pervasive cognitive deficits which are unresponsive to antipsychotic medications. Physical exercise has been shown to improve cognitive functioning in people with schizophrenia, although the mechanisms for this are unclear. We conducted a systematic review of all exercise intervention studies which reported changes in brain structure, connectivity or peripheral biomarkers which could underlie cognitive improvements from exercise in schizophrenia. An electronic database search was conducted on 22 September 2016 using keywords relevant to exercise and neurocognition in schizophrenia. The search returned 2342 articles. Sixteen were eligible for inclusion, reporting data from 14 independent trials of 423 patients with schizophrenia. Seven studies used neuroimaging to examine the impact of exercise on brain structure and connectivity in schizophrenia, whereas seven other studies examined peripheral biomarkers to assess the effects of exercise. Imaging studies collectively indicated that exercise can increase brain volume in people with schizophrenia, although the regions which responded to exercise varied across studies. Most biomarker studies assessed the effects of exercise on serum levels of BDNF. Several studies found significant increases from exercise along with positive correlations between BDNF and cognitive enhancements (indicating a mechanistic link), although other studies did not observe this relationship. In conclusion, the cognitive benefits of exercise in schizophrenia may be due to exercise stimulating neurogenesis, perhaps by up-regulating BDNF, although current evidence is insufficient to draw definitive conclusions. Further exploration of the pro-cognitive mechanisms of exercise in schizophrenia would inform the development of optimal interventions for reducing cognitive impairments in this population.
Linked Articles
This article is part of a themed section on Pharmacology of Cognition: a Panacea for Neuropsychiatric Disease? To view the other articles in this section visit http://onlinelibrary.wiley.com.bibliotheek.ehb.be/doi/10.1111/bph.v174.19/issuetoc
Abbreviations
-
- BDNF
-
- brain derived neurotrophic factor
-
- CRP
-
- C-reactive protein
-
- HIIT
-
- high-intensity interval training
-
- IGF-1
-
- insulin-like growth factor 1
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets | |
| TNF-α |
- These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Schizophrenia is a serious mental disorder which has severe personal, social and economic consequences (Vos et al., 2015). The disorder affects around 1% of the population and causes a financial burden of £11.8 billion per annum in the UK alone (Schizophrenia Commission, 2012). Schizophrenia is characterized by both ‘positive symptoms’ (such as delusions and hallucinations) and ‘negative symptoms’ (low motivation and social withdrawal).
In addition to these aspects of the illness, schizophrenia is associated with pervasive cognitive deficits, including reduced short-term memory, slow processing speed and poor comprehension of social situations (Green et al., 2000; Green and Horan, 2012). These deficits are present from the onset of illness and persist over time to cause much of the socio-occupational disability associated with the disorder, while also impairing functional recovery (Green et al., 2000). Furthermore, the cognitive symptoms of schizophrenia do not respond to antipsychotic medications, and other pharmacological approaches towards resolving these deficits have had little success to date (Keefe et al., 2013).
Nonetheless, physical exercise is one intervention which could treat cognitive deficits of schizophrenia and thus facilitate functional recovery. A recent meta-analysis of 10 controlled trials with 385 patients found that aerobic exercise significantly improves overall cognition in schizophrenia, with particularly large effects on working memory, attentional processes and social cognition (Firth et al., 2016a). This corresponds with earlier research demonstrating benefits from exercise for cognitive functioning in aging populations, healthy adults and those with dementia (Smith et al., 2010; Erickson et al., 2011; Hötting and Röder, 2013).
Despite these promising findings, there is little understanding of the neurobiological mechanisms through which exercise may exert these effects. Previous research has suggested that the cognitive benefits of exercise are a result of up-regulating neurotrophic factors which stimulate neurogenesis (such as ‘brain-derived neurotrophic factor’; BDNF) (Vaynman et al., 2004; Huang et al., 2014), reducing neuroinflammation, which can impair neuronal signalling and neuronal growth (Cotman et al., 2007); increasing the size of grey and white matter structures in the brain; or improving the connectivity between brain areas (Colcombe et al., 2006; Voss et al., 2013; Best et al., 2015; Svatkova et al., 2015). Understanding the processes through which increasing physical activity might improve cognition is important for designing optimal exercise interventions and for developing new treatments that can target these same pathways. However, the mechanisms which underlie cognitive improvements from exercise in schizophrenia are under-researched. Although individual studies have examined individual mechanistic factors which could contribute to the cognitive enhancements observed following exercise interventions in schizophrenia (Pajonk et al., 2010; Kimhy et al., 2015), there has been no systematic review of the evidence in this area to date.
Therefore, the purpose of this review was to identify all exercise intervention studies in schizophrenia which have examined neurobiological variables that may underlie the cognitive effects, evaluate their findings and compare the results. In this way, we aimed to provide further insight into the mechanisms through which exercise can reduce cognitive deficits in schizophrenia, along with examining the relative effects of different types of exercise on the neurobiological factors which may underlie cognitive enhancement.
Search strategy
We conducted an electronic database search of Ovid MEDLINE, PsycINFO, Embase, the Cochrane Central Register of Controlled Trials, the Health Technology Assessment Database, AMED (Allied and Complementary Medicine) and HMIC Health Management Information Consortium from inception on 22 September 2016. The keyword search terms used were as follows: ‘schizo*’ or ‘psychosis’ or ‘psychotic’ and ‘exercise’ or ‘physical activity’ or ‘fitness’ or ‘aerobic’ or ‘resistance training’ and ‘neuro*’ or ‘cogniti*’. Google Scholar and the reference lists of retrieved articles were also searched in order to identify any additional relevant publications.
Eligibility criteria
Articles were independently screened against eligibility criteria by two authors (J.F. and J.C.) independently. Any disagreements were resolved through discussion. Eligible articles were exercise intervention studies published in peer-reviewed journals that were conducted in patient samples of which at least 80% had received a clinical diagnosis of a non-affective psychotic disorder (such as schizophrenia) or were being treated for first-episode psychosis. In order to be included, studies must have examined neurobiological mechanisms pre- and post-intervention that could theoretically account for exercise-induced changes in cognitive functioning among people with psychotic disorders. This included (i) blood measures of neurotrophins, inflammatory cytokines or oxidative stress markers which could influence brain structure and functioning among patients; (ii) structural imaging data on global and region-specific brain volume and white matter integrity; and (iii) functional neuroimaging studies examining the effects of exercise on the neural circuitry which may underlie cognitive functions.
For the purpose of this review, exercise was defined as structured and repetitive physical activity that has an objective of improving or maintaining physical fitness (Caspersen et al., 1985). Studies which incorporated exercise within a broader multi-aspect intervention were also eligible for inclusion provided that the exercise component was clearly defined and consisted of structured physical activity provided on at least a weekly basis (rather than just exercise advice). Interventions using only yoga or tai chi were excluded from the analyses, as these may produce improvements in cognition through mechanisms which are independent from physical activity (Behere et al., 2011). Case studies, review articles and non-English language articles were also excluded.
Data extraction and synthesis
A systematic data extraction form was developed and used to extract the following from each study:
Study details: including sample size, mean age of participants, patient diagnosis/classification (i.e. long term or first episode) and trial design.
Exercise intervention: length (weeks), frequency (sessions per week), duration (session length in min), exercise type (e.g. aerobic, resistance or combined), training protocol outline and control/comparator condition details.
Cognitive outcomes: including the effect size (where reported) and statistical significance of change in cognitive performance on any neuropsychological task (or task battery) in the exercise condition.
Neurobiological changes: any reported changes in the mechanistic factors (as specified above) which could relate to or account for the cognitive-enhancing effects of exercise interventions for schizophrenia.
Search results
The full article screening and selection process is detailed in Figure 1. The initial database search was performed on 22 September 2016. The search returned a total of 2342 results, which was reduced to 1748 after duplicates were removed. At the title and abstract screening stage, 1707 articles were excluded for not meeting the eligibility criteria. Full-text versions for 42 articles were retrieved. Of these, 11 articles were eligible for inclusion. An additional search of the reference lists and Google Scholar identified a further five articles which were eligible for inclusion, although one of these reported data from the studies was identified in the main search. Thus, 16 articles, reporting data from 14 independent studies, were included in this review.
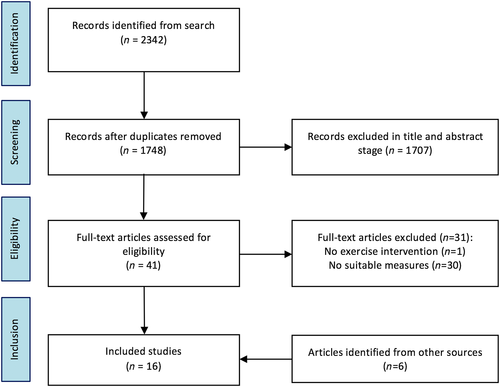
Included studies and participant details
Study details are displayed in Tables 1 and 2. Across the 14 trials, data were available from a total of 423 patients: 370 with long-term schizophrenia/schizoaffective disorder (11 studies) and 53 with first-episode psychosis (three studies). The mean age was 41.4 years (range = 20.2–58.9 years). Nine studies were randomized controlled trials, three were controlled clinical trials and two were single-arm studies.
| Exercise (n) | Control (n) | Mean age (years) | Design | Exercise intervention | Cognitive outcomes of exercise | Exercise effects on brain structure and connectivity | |
|---|---|---|---|---|---|---|---|
| Lin et al., 2015 | 17 | 13 | 24.9 | RCT comparing 12 weeks of aerobic exercise to yoga and usual treatment. | 45–60 min of treadmill walking and stationary cycling at 50–60% VO2 max. Three sessions per week. |
↑Verbal acquisition P = 0.02, d = 0.83. ↑Verbal retention P = 0.016, d = 0.56. ↑Forward digit span P = 0.014, d = 0.59. ↑Backwards digit span P < 0.01, d = 1.08. NC Stroop task. |
Hippocampal grey matter volume was only increased by the exercise condition (F = 7.52, P = 0.01), withthe largest increases in the left hippocampus (F = 5.13, P = 0.03). Yoga or usual treatment did not produce any change. There was no correlation between improved cognition and increases in physical fitness (VO2 max r = 0.07, P = 0.42). |
| Malchow et al., 2015a; Malchow et al., 2015b | 22 | 21 | 36.5 | RCT comparing 12 weeks of aerobic exercise to table football. Both groups augmented with cognitive training. Assessments at 6 and 12 weeks. | 30 min of stationary cycling at gradually increasing intensity. Three sessions per week. |
From 6–12 weeks: ↑Wisconsin card sorting P = 0.008, Z = −2.6. ↑Verbal STM P = 0.03, +10.2%. ↑Verbal LTM P = 0.03, +12.7%. NC Trail-Making Task. |
Increased grey matter volume in left anterior temporal lobe after 3 month exercise intervention compared with baseline (x = −48, y = 0, z = −26; P = 0.05) No significant changes in bilateral hippocampal volumes (in medial temporal lobe). |
| Pajonk et al., 2010; Falkai et al., 2013 | 8 | 8 | 35 | RCT of 12 week aerobic exercise versus table football control group. | Three days of 30 min stationary cycling per week, at an individually defined intensity that was gradually increased over the intervention. |
↑Verbal STM P = 0.04, d = 1.19. NC verbal LTM. NC visuospatial STM. |
Hippocampal volume in the exercise group increased by 12%, significantly more than that in the control condition (F = 13.8, P = 0.002). Exercise group also had a 35% increase in NAA : Cr ratio in the hippocampus. Increases in cardiorespiratory fitness were correlated with increased hippocampal volume (r = 0.83; P = .01) and improved verbal STM (r = 0.51; P = 0.05). For cortical regions, there was no overall effect from exercise, and no correlation with improved cardiorespiratory fitness. |
| Rosenbaum et al., 2015 | 5 | 0 | 20.2 | Single-arm study of 12 week aerobic exercise. | Twice weekly, 45 min sessions of stationary cycling at 65% heart rate of their VO2 max. |
Spatial span: +15%, ns. Verbal learning: −17%, ns. |
No significant change in hippocampal volume ↑0.4%; ↑33.0 mm3; t = 0.5, ns, despite significant improvements in VO2 max (P = 0.02). |
| Scheewe et al., 2013 | 18 | 14 | 30 | RCT of 6 month exercise programme versus occupational therapy. | Twice weekly combined aerobic–resistance training: 40 min of cycling/treadmill/elliptical at up to 75% of max heart rate, then 20 min of resistance training. | N/R |
No main effect from exercise on global brain volume, hippocampal volume or cortical thickness. Improvements in cardiorespiratory fitness (peak wattage) were significantly associated with improvements in brain structure, including increased total cerebral matter volume (0.164 mL·W−1; P = 0.045), decreased lateral ventricle (0.018 mL·W−1; P = 0.035), decreased third ventricle volume (0.0018 mL·W−1; P = 0.013), cortical thickening in the left hemisphere (t = 2.29, P = 0.024), and, at trend-level significance, cerebral grey matter increases (0.159 mL·W−1; P = 0.059). |
| Svatkova et al., 2015 | 16 | 17 | 30.1 | RCT comparing 6 month exercise to usual treatment. | Twice weekly combined aerobic–resistance training: 40 min of cycling/treadmill/elliptical at up to 75% of max heart rate, then 20 min of resistance training. |
↑Global IQ P = 0.33, +4.1 points. |
Exercise significantly improved white matter integrity in fibre tracts implicated in motor functioning. Overall improvement in white matter integrity held a significant correlation with cardiorespiratory fitness (VO2 peak; r = 0.27, P = 0.016). However, there were no significant associations between fitness and IQ or between brain changes and IQ. |
| Takahashi et al., 2012 | 13 | 10 | 41.9 | CCT comparing 3 months healthy lifestyle programme to usual treatment. | Exercise module of the lifestyle programme was delivered twice daily for 30–60 min, comprising walking and jogging, muscle-stretching exercises and sport group (basketball). | N/R |
Exercise intervention significantly increased brain activation in the extrastriate body area of the posterior temporal cortex when watching basketball clips (peak: x = 42, y = 74, z = 4, Z score = 4.12). No change in control condition. Greater extrastriate body area activation was associated with greater reduction in the general symptoms subscale of the PANSS (r = 0.78, P = 0.002), but not for positive or negative symptom subscales (all P > 0.05). |
- CCT, controlled clinical trial; IQ, intelligence quotient; LTM, long-term memory; NAA : Cr, n-acetylaspartate to creatine ratio; NC, no change; N/R, not reported; PANSS, Positive and Negative Syndrome Scale; RCT, randomized clinical trial; STM, short term memory; ns, not significant.
| Exercise (n) | Control (n) | Mean age (years) | Design | Exercise intervention | Cognitive outcomes of exercise | Exercise effects on biomarkers | |
|---|---|---|---|---|---|---|---|
| Cassilhas et al., 2015 | 21 | 13 | 33.3 | RCT of 20 weeks resistance training versus combined aerobic–resistance training versus low-intensity control group. | 60 min, twice weekly, of either (i) progressively difficult resistance exercises targeting large muscle groups; (ii) half-resistance exercises, half treadmill running; or (iii) control group of very low-weight resistance exercise with slow treadmill walking. | N/R | No change in BDNF or IGF-1 |
| Heggelund et al., 2011 | 12 | 7 | 33.5 | CCT comparing 8 weeks of HIT to computer games. | Sessions consisted of 4 × 4 bouts of high-intensity treadmill running at 85–95% max heart rate, interspersed with 3 min rest periods (walking). | N/R | Hs-CRP (mg·L−1) in the exercise group went from 8.09 (s.d. = 18.15) to 2.67 (s.d. = 2.11) after 8 weeks. In the control group, Hs-CRP went from 4.41 (s.d. = 5.34) to 4.26 (s.d. = 5.09). No significant difference between groups. |
| Ho et al., 2016 | 51 | 49 | 54.9 | Three-arm RCT comparing 12 weeks of exercise or tai chi to wait list control. | 60 min of low-intensity activity designed to match physical exertion of tai chi (50–60% maximal oxygen consumption), for example, stretching, walking and mild weight training. |
↑Forward digit span P < 0.05, d = 0.42. NC backwards span. |
Salivary cortisol in the exercise group significantly increased significantly more than that in the waitlist control (P < 0.05, d = 0.46). |
| Kim et al., 2014 | 24 | 12 | 49.4 | 12 week RCT of combined aerobic–resistance training versus low-intensity control. | 3 days·week−1 of 25 min resistance exercise (using elastic bands) plus 25 min treadmill walking at 60% max heart rate. | N/R |
BDNF increased following the exercise programme (13.2 ng·mL−1 to 16.0 ng·mL−1, P = 0.007), significantly more than the control condition. Increases in BDNF correlated with improvements in cardiovascular fitness (YMCA step test; r = 0.404, P = 0.035) and leg strength (r = 0.446, P = 0.021), but not with grip strength (r = 0.03, P = 0.45). |
| Kimhy et al., 2015 | 13 | 13 | 36.9 | RCT comparing 12 weeks of aerobic exercise to usual treatment. | 60 min at 60–75% VO2 peak of multi-modal exercise including treadmill running, elliptical training and interactive video gaming. Three sessions per week. | ↑Global cognition P = 0.031, d = 0.93. |
BDNF increased by 11.0% in the exercise group versus 1.9% in the usual treatment, but with no significant difference between groups (d = 0.3, P = 0.46). Regression analyses found that improvements in cognitive performance were predicted by both increased BDNF (F = 4.11, P = 0.05) and increased fitness (F = 8.16, P = 0.009). |
| Kuo et al., 2013 | 33 | 0 | 37.8 | Single-arm study of 10 week healthy lifestyle programme. | Weekly group exercise class (1 h), plus encouragement to engage in regular low-intensity activity and moderate-intensity activity (fast walking or cycling) five times per week. | N/R |
BDNF increased from 4.5 ng·mL−1 to 9.1 ng·mL−1 after the multi-element programme (P < 0.001). There was no change in CRP, IL-6 or TNF-ɑ. Increases in BDNF were strongly correlated with reductions in bodyweight (r = 0.773, P < 0.001) and BMI (r = 0.63, P < 0.001). |
| Nuechterlein et al., 2016 | 7 | 9 | 22.7 | CCT of 10 weeks of aerobic exercise combined with cognitive training versus cognitive training alone. | Four times weekly aerobic exercise at 60–80% of max heart rate, using 30–45 min bodyweight workout videos (doing lunges, squats, pushups, etc.) |
↑Global cognition P: N/R, d = 0.96. ↑Social cognition P: N/R, d = 1.3. ↑Working memory P: N/R, d = 1.0. ↑Processing speed P: N/R, d = 0.76. ↑Attention/vigilance P: N/R, d = 0.66. |
BDNF increased from baseline to follow-up in the exercise group (mean change: 4.22 ng·mL−1 ), with an accompanying change in cardiorespiratory fitness (d = 0.94) |
- BMI, body mass index; CCT, controlled clinical trial; D, Cohen's d; Hs-CRP, high-sensitivity CRP; NC, no change; N/R, not reported; RCT, randomized clinical trial; HIT, High Intensity Training; s.d., standard deviation; YMCA, Young Men's Christian Association.
A total of 247 patients were allocated to exercise interventions and 176 to comparison conditions. Most exercise interventions lasted 12 weeks (eight studies), with others lasting 6 months (two studies), 10 weeks (two studies), 8 weeks (one study) or 20 weeks (one study). Exercise interventions included moderate aerobic training alone (Pajonk et al., 2010; Takahashi et al., 2012; Kimhy et al., 2015; Lin et al., 2015; Rosenbaum et al., 2015; Malchow et al., 2016), combined aerobic and resistance training (Scheewe et al., 2013; Kim et al., 2014; Cassilhas et al., 2015; Svatkova et al., 2015), low-intensity exercise with some aerobic activity (Kuo et al., 2013; Ho et al., 2016), high-intensity interval training (HIIT) (Heggelund et al., 2011) or bodyweight workout videos (Nuechterlein et al., 2016): two of the studies embedded exercise within broader ‘lifestyle programs’ for encouraging healthy habits (Takahashi et al., 2012; Kuo et al., 2013) and two combined exercise with cognitive remediation therapy (Malchow et al., 2016; Nuechterlein et al., 2016) but controlled for this in the comparator condition. Other control conditions included occupational therapy (Scheewe et al., 2013), tai chi (Ho et al., 2016), table football (Malchow et al., 2016), yoga (Lin et al., 2015), video games (Heggelund et al., 2011) and usual treatment (Takahashi et al., 2012; Lin et al., 2015; Svatkova et al., 2015).
Seven studies used neuroimaging techniques to assess the effects of exercise in schizophrenia, and seven others used blood/salivary biomarkers to assess physiological response to exercise interventions. No studies used both neuroimaging and biomarker analysis.
Findings from imaging studies
The full details and outcomes of all studies examining the effects of exercise on brain structure and connectivity in people with schizophrenia are presented in Table 1. Additionally, the findings of these studies are systematically summarized below.
Five studies examined the effects of exercise on brain volume in patients with schizophrenia. Each of these also reported changes in the hippocampus from exercise. Two studies (Pajonk et al., 2010; Lin et al., 2015) found that 12 weeks of aerobic exercise delivered three times per week increased hippocampal volume more than the other study conditions (which included yoga, table football and waitlist control). Increased hippocampal volume corresponded with a significant increase in short-term memory. Furthermore, both studies reported indications that these increases in brain volume were linked to improved cardiorespiratory fitness. Pajonk et al. (2010) reported a strong correlation between increases in hippocampal size and maximal exercise capacity (r = 0.83, P = 0.01) and (Lin et al., 2015) found trend-level improvements in cardiorespiratory fitness only occurred in the exercise group (which was also the only study condition to increase hippocampal volume).
However, conflicting evidence for hippocampal growth following exercise is presented by Rosenbaum et al. (2015) and Malchow et al. (2016), both of which also used 12 weeks of aerobic exercise with three sessions per week. These studies failed to observe any significant increases in hippocampal volume from exercise, despite successfully increasing fitness (Rosenbaum et al., 2015) and short-term memory (Malchow et al., 2016). Nonetheless, Malchow et al. (2016) did find a significant increase in left anterior lobe volume (P = 0.05).
Additionally, the study by Scheewe et al. (2013), which used combined aerobic–resistance training twice weekly for 6 months, also found no change in hippocampal volume or any of the other brain regions studied. However, they did find that increased cardiorespiratory fitness over the 6 months was predictive of changes in brain structure, including increases in total cerebral volume (P = 0.045), decreases in the lateral (P = 0.035) and third ventricle (P = 0.013) and left hemisphere cortical thickening (P = 0.024).
Svatkova et al. (2015) used the same training protocol as Scheewe et al. (2013) to examine the effects of exercise on white matter integrity in patients with schizophrenia. Results showed a significant improvement in various fibre tracts implicated in motor functioning. Again, these improvements were significantly associated with increased cardiorespiratory fitness over the intervention period (P = 0.016). However, the improvement in global cognitive functioning (measured using IQ scores) was not statistically significant and did not correlate with neurological changes.
Finally, Takahashi et al. (2012) was the only study to examine changes in brain activation from exercise in schizophrenia, using fMRI techniques. The intervention incorporated aerobic exercise and daily participation in basketball groups as part of a ‘healthy lifestyle programme’. Participants' brain activation while watching sports-related videos and non-sport videos was measured before and after the 3 month programme and compared with a group who were not participating in the lifestyle programme. Brain activation increased in the extrastriate body area of posterior temporal cortex when watching sport-related videos following the 3 month intervention, whereas no change was observed in the control condition. The degree of change in the intervention group strongly predicted improvements in general symptomatology (r = 0.78, P < 0.01).
Mechanistic findings from biomarker studies
The details and outcomes of all studies examining the effects of exercise on cognitive biomarkers in people with schizophrenia are presented in Table 2. Additionally, the effects of exercise on each biomarker are summarized below.
The most widely studied biomarker was BDNF. This was examined in five studies with a total of 145 participants with schizophrenia. Of these, three interventions, which combined aerobic exercise with cognitive training (Nuechterlein et al., 2016), resistance training (Kim et al., 2014) and healthy lifestyle advice (Kuo et al., 2013), reported increases in BDNF. However, Cassilhas et al. (2015) found no effects on BDNF from 20 weeks of either resistance training or combined aerobic–resistance training. Kimhy et al. (2015) also failed to observe a main effect of exercise on BDNF, as the moderate increases in BDNF observed in this small sample (n = 26) fell short of statistical significance.
Three studies explored the factors associated with exercise-induced improvements in BDNF using correlation analyses. Kimhy et al. (2015) found a significant main effect of exercise on global cognition and further reported that increases in BDNF predicted 14.6% of the improvements in cognitive performance from exercise, while increases in cardiorespiratory fitness accounted for 25.4% of the improvements observed. Kim et al. (2014) also found that increases in BDNF were significantly associated with improvements in cardiorespiratory fitness (r = 0.404, P = 0.035). Finally, Kuo et al. (2013) found that elevation in BDNF was positively associated with reductions in bodyweight and BMI following the 10 week multi-component lifestyle programme (P < 0.001).
Two studies examined changes in inflammatory markers following exercise interventions. Heggelund et al. (2011) studied changes in c-reactive protein (CRP) following 8 weeks of HIIT in comparison to a video game control condition. Although there was a 66% reduction in CRP from the exercise intervention and no change in the control condition, the difference between the two groups was not statistically significant. Kuo et al. (2013) also found no significant changes in CRP, IL-6 or TNF-ɑ from their 10 week lifestyle intervention.
Changes in insulin-like growth factor 1 (IGF-1) and salivary cortisol following exercise interventions were reported in the following studies respectively. Cassilhas et al. (2015) found no change in IGF-1 from a 20 week combined aerobic–resistance training programme. Ho et al. (2016) found that low-intensity exercise moderately increased in salivary cortisol (P < 0.05) while also increasing working memory as measured by the forward digit span test (P < 0.05).
Discussion
This review aimed to identify and evaluate all existing studies which have examined the neurobiological mechanisms through which exercise may reduce cognitive deficits in people with schizophrenia. Whereas a previous meta-analysis has demonstrated the efficacy of exercise for improving cognition in schizophrenia (Firth et al., 2016a), this is the first systematic review of the mechanisms which may underlie these effects. Of the 14 eligible independent studies, seven investigated the effects of exercise on brain structure and functioning and seven investigated the effects on peripheral markers, which may indicate or produce cognitive improvements.
Exercise and/or improved cardiorespiratory fitness was associated with increased brain volumes in certain regions, for all but one study that assessed structural brain changes (which may be due to the null study having small sample size of only five participants; Rosenbaum et al., 2015). These findings are consistent with the increased brain volume found in response to exercise in other populations (Erickson et al., 2011; Voss et al., 2013). Thus, stimulation of neurogenesis could be one mechanism through which exercise may have its pro-cognitive effects.
However, the effect of exercise on specific brain regions was inconsistent across studies. For instance, two studies found significant increases in hippocampal volume (Pajonk et al., 2010; Lin et al., 2015). These changes were observed in the schizophrenia group, but not in the healthy control sample (Lin et al., 2015). This suggests that exercise could attenuate the deterioration in hippocampal volume that can occur in the early phases of schizophrenia (Velakoulis et al., 2006). Indeed, these findings are similar to those found in aging populations, where neuronal loss in the hippocampus was also attenuated through exercise (Erickson et al., 2011). However, two other studies using an identical training protocol failed to replicate these findings, despite significantly increasing cognitive performance and grey matter volume in other brain regions (Malchow et al., 2016). The same variability applied to the link between cardiorespiratory fitness and neurological improvements. Whereas some studies found no relationship of exercise with cortical regions, others did find significant correlations between increased fitness and hippocampal volume (along with short term memory, which is a cognitive function often associated with this brain area) (Pajonk et al., 2010; Falkai et al., 2011). Another study found significant associations between fitness improvement and cortical thickness, but with no association with hippocampal change (Scheewe et al., 2013). Given these inconsistencies, further evidence is required to determine if and how the cognitive benefits of exercise relate to growth in particular brain regions. Nonetheless, it should be noted that studies which compared the effects of exercise across hemispheres did consistently find that the left hemisphere was more sensitive to exercise-induced improvements than the right (Scheewe et al., 2013; Lin et al., 2015; Malchow et al., 2016).
Effects of exercise on biomarkers were also inconsistent across studies, including those measuring BDNF. BDNF is the most abundant neurotrophic factor in humans and is up-regulated in response to exercise (Szuhany et al., 2015). It predicted cognitive benefits of exercise in healthy populations (Griffin et al., 2011). Animal studies have indicated a critical role of BDNF in exercise-related cognitive enhancement, as blocking the BDNF channels prevented the improvements usually observed in rats from regular physical activity (Vaynman et al., 2004). However, the role of BDNF in exercise treatments for schizophrenia has yet to be established. Whereas serum BDNF levels did notably increase in four of the five studies, which examined this possible mechanism (Kuo et al., 2013; Kim et al., 2014; Kimhy et al., 2015; Nuechterlein et al., 2016), only two observed a statistically significant improvement (Kuo et al., 2013; Kim et al., 2014). However, this may have been due to the small sample sizes of n = 13 and n = 4 in the intervention arms of these studies. Nonetheless, increases in BDNF did hold significant associations with exercise-induced improvements in fitness, body composition and most importantly cognitive functioning in each study which examined these relationships (Kuo et al., 2013; Kim et al., 2014; Kimhy et al., 2015). Previous meta-analyses have also found that populations with depression are more sensitive to up-regulation of BDNF in response to exercise than healthy subjects (Szuhany et al., 2015).
Two studies found no effect of exercise on inflammatory markers in patients with schizophrenia (Heggelund et al., 2011; Kuo et al., 2013). This is an interesting finding given that patients with schizophrenia show low-grade systemic inflammation (Potvin et al., 2008), which may affect cognitive functioning (Dickerson et al., 2007), and exercise is known to have anti-inflammatory effects in other populations (Petersen and Pedersen, 2005). However, levels of circulating pro-inflammatory cytokines in the blood are confounded by many factors, including smoking and obesity, both common in schizophrenia (McCreadie, 2003), and do necessarily reflect inflammation in the brain (Bergink et al., 2014). Thus, the positive effects of exercise in reducing neuroinflammation may not be detected using these peripheral measures. Future research using positron emission tomography to assess microglial activation and magnetic resonance spectroscopy to measure brain glutathione could derive more valid information on the anti-inflammatory and anti-oxidative effects of exercise in schizophrenia. If such studies did find a reduction of neuroinflammation and oxidative stress in response to exercise, this could inform the development of interventions targeting these processes in order to reduce cognitive dysfunction.
One limitation in this review is the substantial variation between studies in the exercise interventions applied, the comparison groups used and the outcomes studied, making it difficult to compare findings across studies. It is possible that the inconsistencies across study findings arose as a result of the differences between the interventions applied. However, whereas this review has focused on pro-cognitive mechanisms from any type of exercise, other recent reviews which have examined neural and cognitive effects of exercise in schizophrenia have also found broad inconsistencies and significant heterogeneity across studies, even when focusing entirely on aerobic exercise interventions (Firth et al., 2016a; Vakhrusheva et al., 2016).
Along with training type, exercise dosage is another factor which may affect the neurobiological changes deriving from physical activity interventions, since studies which use greater amounts of exercise also tend to result in greater cognitive improvements among patients with schizophrenia (Firth et al., 2016a). Exercise intensity may also be a critical factor, as intervention studies have observed that the patients who achieve greater adherence to moderate-to-vigorous exercise are more likely to experience cognitive enhancements (Kimhy et al., 2016; Firth et al., 2016b). Further mechanistic research is needed to establish the importance of exercise dosage versus exercise type for designing optimal programs for cognitive enhancement.
However, it is also important to consider that people with severe mental health problems engage in less moderate and vigorous activity than the general population (Schuch et al., 2016; Stubbs et al., 2016; Vancampfort et al., 2016), due to a combination of psychological and social barriers (Firth et al., 2016c). Previous studies have shown that providing exercise facilities and advising patients to be physically active fails to increase physical activity (Archie et al., 2003). Nonetheless, taking into account the individual motivations of patients and their preferences for exercise, in order to create personalized training programs, does enable people with schizophrenia to engage in sufficient amounts of exercise (Firth et al., 2016b; Firth et al., 2016d).
A further limitation in this review is heterogeneity of samples in terms of age and duration of illness, as participants ranged between 20 and almost 60 years old, and we included three studies of first-episode psychosis, along with 11 of long-term schizophrenia. There is a possibility that findings may not generalize between these two groups, since other cognitive enhancement interventions have been found to be more effective in first-episode psychosis than in long-term illness (Bowie et al., 2014). Indeed, the two controlled trials of exercise in first-episode psychosis found improvements in cognitive functioning across various domains, accompanied by increased hippocampal volume (Lin et al., 2015) and serum BDNF (Nuechterlein et al., 2016). This is consistent with the findings that the early stages of schizophrenia are associated with more inflammation, compared with chronic illness (Miller et al., 2011; Upthegrove et al., 2014). Furthermore, the early stages of illness may be the optimal time period to target cognitive deficits in order to improve real-world functioning and facilitate full recovery (Bowie et al., 2014; Cotter et al., 2014).
In conclusion, there is preliminary evidence that the cognitive benefits of exercise for schizophrenia are accompanied by improvements in brain structure and connectivity, although the mechanisms of exercise-induced cognitive improvements cannot, as yet, be attributed to growth in any particular brain area. Similarly, although increase in BDNF is a promising finding for explaining the cognitive effects of exercise, information is too limited to draw any firm conclusions. Furthermore, peripheral markers of inflammation have yet to show any relationship with exercise in schizophrenia. Future studies which directly compare alternative physical activity regimes (differing in terms of either dosage or exercise modality) are needed. Additionally, there is currently a complete lack of intervention studies which combine neuroimaging with biomarker measurement and fitness assessment in order to elucidate the neurobiological pathways through which exercise enhances cognition in schizophrenia. This is an important area for future research to gain further insight into pro-cognitive mechanisms of exercise. This in turn would lead to optimal intervention development and exercise prescription for reducing cognitive deficits in schizophrenia and thus improving functional recovery for patients.
Conflict of interest
The authors declare no conflicts of interest.