Comparison of the HAS-BLED versus ORBIT scores in predicting major bleeding among Asians receiving direct-acting oral anticoagulants
The authors confirm that the PI for this paper is Surakit Nathisuwan and that he had direct clinical responsibility for patients.
Abstract
Aims
This study aimed to evaluate the performance of HAS-BLED and ORBIT scores in predicting bleeding risk among Asian patients with nonvalvular atrial fibrillation (NVAF) using direct-acting oral anticoagulants (DOACs).
Methods
A retrospective chart review was conducted among adult patients receiving DOACs for ≥6 months during January 2013 to December 2017 in 10 tertiary care hospitals in Thailand. The area under the receiver operating curve (AUROC) method or C-statistic was used to test the diagnostic accuracy for bleeding risk classification of HAS-BLED and ORBIT scores. The predictive performances of the two scores were compared using DeLong's method.
Results
A total of 961 NVAF patients, 52.5% warfarin-naïve and 47.5% warfarin-experienced, with mean age of 74.25 ± 10.08 years, were included in the analysis. Mean HAS-BLED and ORBIT scores of the cohort were 1.98 ± 1.10 and 2.37 ± 1.71, respectively. During the mean follow-up time of 1.55 ± 1.13 years, 34 patients experienced major bleeding (2.28 events/100 patient-year). For the overall cohort, both the HAS-BLED and ORBIT scores showed similarly moderate predictive performance on bleeding with C-statistic (95% confidence interval) of 0.65 (0.57-0.74) and 0.64 (0.56-0.71), respectively. There was no statistical significance between the two scores (P = .62). Analysis based on the status of previous warfarin use was consistent with the overall cohort. Based on the calibration analysis, both HAS-BLED and ORBIT scores possessed moderate ability to identify those who experienced major bleeding from those who did not.
Conclusion
Both HAS-BLED and ORBIT bleeding risk scores had moderate predictive performance in Asian NVAF patients receiving DOACs.
What is already known about this subject
- Little is known regarding the performance of common bleeding risk scores among Asian patients with nonvalvular atrial fibrillation using direct-acting oral anticoagulants.
What this study adds
- Both the HAS-BLED and ORBIT scores showed similarly moderate predictive performance on bleeding when applied to an Asian population. These scores possessed moderate ability to identify those who experienced major bleeding from those who did not.
1 INTRODUCTION
Direct-acting oral anticoagulants (DOACs) have become the standard of care for stroke prevention in nonvalvular atrial fibrillation (NVAF), being supported by guidelines and increasing real-world clinical practice data.1-4 These agents have favourable pharmacokinetics and pharmacodynamics profiles compared to vitamin K antagonists (VKA, eg, warfarin). Data from both randomized trials and real-world studies have consistently shown that these pharmacological advantages translate into better clinical outcomes.5, 6 For developing countries, DOAC usage is on the rise and will most likely increase exponentially with the generic availability of some of these agents. Despite their advantages, DOACs still have some risks, particularly major bleeding.7 Previous studies suggested that the Asian population is at a higher risk of bleeding compared to other racial groups.8, 9 As a result, the ability to accurately predict the bleeding risk of an individual patient may be beneficial, particularly among the Asian population, who have higher bleeding risk.
There are a number of bleeding risk scores available in the published literature. Among clinical risk scores, the HAS-BLED score is widely used and validated in various patient populations and with various types of oral anticoagulants.10, 11 The ORBIT score has also been used, and more recently recommended in the UK National Institute for Health and Care Excellence (NICE) guideline.12 For the Asian population, a recent study conducted in the Japanese NVAF population using DOACs showed that both scores have moderate discriminatory performance.13 Other studies conducted in the Asian population had too few DOACs users, short follow-up time or a limited number of bleeding events.14, 15 This study therefore aimed to evaluate the predictive performance of HAS-BLED and ORBIT scores in predicting bleeding risk among Thai NVAF patients receiving DOACs. Since previous study indicated that the risk of bleeding may differ based on the history of warfarin exposure,16 we conducted analysis in three ways, including the overall cohort, warfarin-naïve and warfarin-experienced patients to observe any potential differences in the performances of the scores based on previous warfarin exposure.
2 METHOD
2.1 Study design
We used data from the recent study by Wattanaruengchai et al for which the original design and the results of the main study have been published previously.17 Briefly, the study was a multicentre study conducted in 10 public, tertiary care hospitals from four provinces (Bangkok, Chiang Mai, Nakhon Nayok and Nakhon Ratchasima) in Thailand to evaluate the appropriateness of DOAC use in mixed indications.
2.2 Study population
Adult patients (≥18 years old) with NVAF who received DOACs for 6 months or more during January 2013 to December 2017 for stroke prevention were used as the study cohort. The follow-up period of this study was from the initiation of DOACs until the occurrence of major bleeding, drug discontinuation, switching of therapy or the end of study period. Data collection was performed through medical chart review then relevant data were extracted into a standardized case record form. Patients using DOACs for indications other than NVAF or off-label use, and patients with missing data on body weight or serum creatinine were excluded from this analysis. The study protocol was approved by the Institutional Review Board of each participating hospital.
2.3 Bleeding risk scores and major bleeding events
The HAS-BLED bleeding risk score comprises abnormal haemoglobin (<13 mg/dL for males and <12 mg/dL for females) or haematocrit (<40% for males and <36% for females), hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), elderly and concomitant use of certain drugs and alcohol.10 The detailed scoring system is listed in Supporting Information Table S1. Risk was classified into two levels for low- (score 0-2) and high- (score ≥3) risk groups. For the evaluation of DOAC, the labile INR criteria of HAS-BLED was set to 0 in all patients except those receiving an overdose of DOAC, where it was set to 1, given that inappropriate DOAC overdosing has been shown to increase the risk of bleeding.17 The ORBIT bleeding risk score comprises five risk factors: age ≥75, anaemia, bleeding history, estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 (calculated by the modification of diet in renal disease equation) and use of antiplatelet drugs.11 While the risk was originally classified into three levels for low- (score 0-2), intermediate- (score 3), and high- (score ≥4) risk groups, we opted to divide patients into two groups, low versus intermediate/high risk. This approach has been taken previously, which allowed direct comparison between the two scores.18 Both scores were calculated at the time of treatment initiation. Major bleeding events were defined according to International Society on Thrombosis and Haemostasis criteria.19
2.4 Statistical analysis
Continuous variables were analysed as means and standard deviations (SD). Categorical variables were analysed as counts and percentages (%). Bleeding outcomes by the HAS-BLED and ORBIT bleeding scores were computed as the rate of events per 100 patient-years. The Kaplan-Meier method was used to estimate the cumulative incidences of bleeding outcome and the Cox proportional hazard model was used to compare the rates of major bleeding among different bleeding risk classifications (low, intermediate and high). The area under the receiver operating curve (AUROC) method or C-statistics was used to test the diagnostic accuracy for bleeding risk classification of HAS-BLED and ORBIT scores. The predictive performances of HAS-BLED and ORBIT were subsequently compared according to a method proposed by DeLong et al.20 We also reported sensitivity, specificity, negative predictive value (NNP) and positive predictive value (PPV) for a binary threshold (high versus not-high bleeding risk) for each bleeding score to assess the performance of each score. The performance prediction model21 was used to assess the discrimination of bleeding risk scores in our cohort compared to the original cohort,10, 11 representing in the C-statistic for discriminative ability (or AUROC). The predictive performance of the HAS-BLED and ORBIT scores was evaluated using calibration plots by 0, 1, 2, 3, ≥4 for both bleeding scores. In addition to the overall population analysis, we also evaluated the performance of the risk scores among warfarin-naïve and warfarin-experienced patients to observe any potential differences in their predictive performances based on previous warfarin exposure. Statistical significance was defined as P < .05. All statistical analysis was performed by using SPSS version 21 and STATA version 14.1.
3 RESULTS
3.1 Baseline characteristics
The baseline characteristics of the NVAF study cohort (N = 961) are presented in Table 1. Mean age was 74.25 ± 10.08 years, 49.7% were male. Mean body weight was 64.60 ± 14.13 kg. Mean eGFR was 64.71 ± 18.77 mL/min/1.73 m2. For types of DOACs, 47.1%, 27.4% and 25.5% received dabigatran, rivaroxaban and apixaban, respectively. The total follow-up time was 1493.04 patient-years (mean ± SD 1.55 ± 1.13 years, median 1.24 [interquartile range (IQR) 0.74-2.08 years]). The mean ORBIT score was 2.37 ± 1.71 with 55.5% and 44.5% of patients at low and intermediate/high risk for bleeding. For HAS-BLED, the mean score was 1.98 ± 1.10 with 68.1% and 31.9% patients at low and high risk for bleeding. Distribution of each risk item for the HAS-BLED and ORBIT bleeding scores in the study cohort is presented in Supporting Information Table S2. For the HAS-BLED score, the most common qualifying risk factors were age (81%), history of prior bleeding (48.7%) and history of prior stroke (34.9%). For the ORBIT score, the most common qualifying risk factors were age (53.1%), insufficient kidney function (41.2%) and anemia (40.8%).
| Characteristics | Total (N = 961) | Warfarin -naïve (N = 505) | Warfarin-experienced (N = 456) | P value |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD (y) | 74.25 ± 10.08 | 74.54 ± 10.55 | 73.92 ± 9.55 | 0.339 |
| <65 | 165 (17.2) | 85 (16.8) | 80 (17.5) | 0.797 |
| ≥65 to <75 | 286 (29.8) | 155 (30.7) | 131 (28.7) | |
| ≥75 | 510 (53.1) | 265 (52.5) | 245 (53.7) | |
| Gender | ||||
| Female | 483 (50.3) | 241 (47.7) | 242 (53.1) | 0.098 |
| Male | 478 (49.7) | 264 (52.3) | 214 (46.9) | |
| Body weight | ||||
| Mean ± SD (kg) | 64.60 ± 14.13 | 64.75 ± 13.52 | 64.45 ± 14.79 | 0.743 |
| <60 | 360 (37.5) | 182 (36.0) | 178 (39.0) | 0.338 |
| ≥60 | 601 (62.5) | 323 (64.0) | 278 (61.0) | |
| Renal function (eGFR) | ||||
| Mean ± SD (mL/min/1.73m2) | 64.71 ± 18.77 | 65.42 ± 18.31 | 63.92 ± 19.26 | 0.216 |
| < 60 | 396 (41.2) | 197 (39.0) | 199 (43.6) | 0.145 |
| ≥60 | 565 (58.8) | 308 (61.0) | 257 (56.4) | |
| Stroke risk score | ||||
| CHA2DS2VASc score (mean ± SD) | 4.11 ± 1.71 | 4.02 ± 1.71 | 4.21 ± 1.70 | 0.077 |
| Comorbidities | ||||
| Hypertension | 773 (80.4) | 420 (83.2) | 353 (77.4) | 0.025 |
| Diabetes | 322 (33.5) | 163 (32.3) | 159 (34.9) | 0.395 |
| Vascular disease | 235 (24.5) | 130 (25.7) | 105 (23.0) | 0.328 |
| Heart failure | 171 (17.8) | 73 (14.5) | 98 (21.5) | 0.004 |
| Previous complications | ||||
| Previous stroke | 335 (34.9) | 158 (31.3) | 177 (38.8) | 0.014 |
| Previous bleeding | 157 (16.3) | 62 (12.3) | 95 (20.8) | <0.001 |
| Concomitant drugs | ||||
| Antiplatelet drugs | 160 (16.6) | 103 (20.4) | 57 (12.5) | 0.001 |
| NSAIDs | 43 (4.5) | 20 (4.0) | 23 (5.0) | 0.417 |
| Systemic steroids | 23 (2.4) | 15 (3.0) | 8 (1.8) | 0.218 |
| DOAC type | ||||
| Dabigatran | 453 (47.1) | 228 (45.1) | 225 (49.3) | 0.428 |
| Rivaroxaban | 263 (27.4) | 143 (28.3) | 120 (26.3) | |
| Apixaban | 245 (25.5) | 134 (26.5) | 111 (24.3) | |
| DOAC appropriateness | ||||
| Label-approved dosing | 656 (68.3) | 339 (67.1) | 317 (69.5) | 0.291 |
| Underdosing | 201 (20.9) | 115 (22.8) | 86 (18.9) | |
| Overdosinga | 104 (10.8) | 51 (10.1) | 53 (11.6) | |
| Bleeding risk scores | ||||
| HAS-BLED score (mean ± SD) | 1.98 ± 1.10 | 1.96 ± 1.11 | 2.00 ± 1.08 | 0.537 |
| Low risk (0-2) | 654 (68.1) | 343 (67.9) | 311 (68.2) | 0.926 |
| High risk (≥3) | 307 (31.9) | 162 (32.1) | 145 (31.8) | |
| ORBIT score (mean ± SD) | 2.37 ± 1.71 | 2.26 ± 1.65 | 2.50 ± 1.77 | 0.050 |
| Low risk (0-2) | 533 (55.5) | 294 (58.2) | 239 (52.4) | 0.195 |
| Intermediate/high risk (≥3) | 428 (44.5) | 211 (41.8) | 217 (47.6) | |
- ACEIs/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; CHA2DS2VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack/thromboembolism history (doubled), vascular disease history (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65-74 years, gender category (female); eGFR, estimated glomerular filtration rate; HAS-BLED, hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile inr, elderly, drug/alcohol usage; NSAIDs, nonsteroidal anti-inflammatory drugs; ORBIT = Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
- a Overdosing: warfarin-naïve 51 cases (dabigatran 31 cases, rivaroxaban 15 cases and apixaban five cases), warfarin-experienced 53 cases (dabigatran 23 cases, rivaroxaban 23 cases and apixaban seven cases).
- ORBIT = Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
3.2 Event rates of major bleeding related to risk stratification
During the follow-up period, there were 34 patients who experienced major bleeding. The overall major bleeding rate was 2.28 events/100 patient-years. The detailed distribution of the score and corresponding major bleeding rates for both HAS-BLED and ORBIT scores of the derivative cohort and the study cohort are shown in Supporting Information Tables S3 and S4. The annualized bleeding rate increased with increasing risk factors with both scoring systems. For HAS-BLED, the rates of major bleeding were 1.28 and 4.2 events/100 patient-years for low and high risk group. For ORBIT, the rates of major bleeding were 1.19 and 3.70 events/100 patient-years for the low- and intermediate/high-risk groups. Based on the Cox regression models, both risk scores were able to discriminate the risk of major bleeding in the overall study cohort, warfarin-naïve and warfarin-experienced patients (Figure 1A-F).
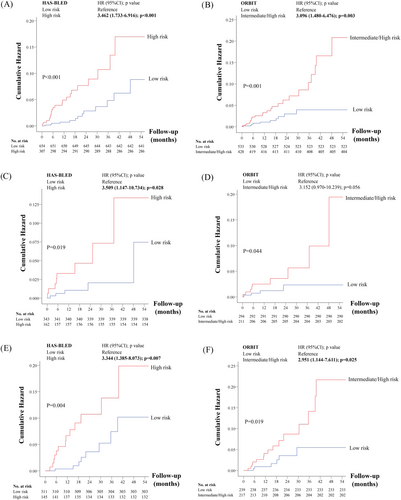
3.3 Discrimination of bleeding risk scores
In the overall cohort, both the HAS-BLED and ORBIT scores showed moderate predictive performance on major bleeding with C-statistics (95% confidence interval [CI]) of 0.65 (0.57-0.74) and 0.64 (0.56-0.71), respectively (Figure 2A). There was no statistical significance between the two scores (P = .62).
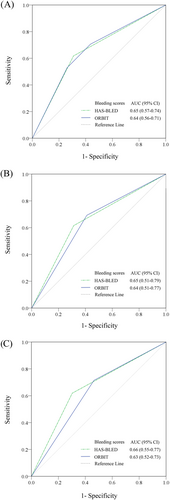
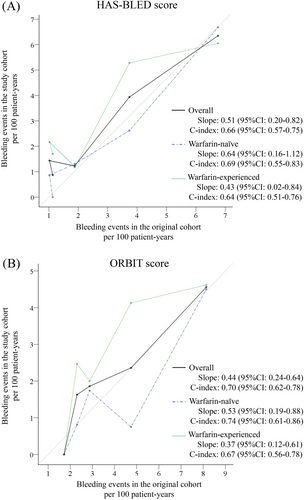
The C-statistics (95% CI) of HAS-BLED vs ORBIT score were 0.65 (0.51-0.79) vs 0.64 (0.51-0.77) for warfarin-naïve patients and 0.66 (0.55-0.77) vs 0.63 (0.52-0.73) for warfarin-experienced patients (Figure 2B,C). Both scores also had comparable sensitivity, specificity, NPV and PPV (Table 2). Based on the calibration analysis, both HAS-BLED and ORBIT scores possessed moderate ability to identify those who experienced major bleeding from those who did not, for the overall cohort, warfarin-naïve or warfarin-experienced patients (Figure 3A,B). We also performed sensitivity analysis by comparing the two risk scores based on type of DOAC, including dabigatran, rivaroxaban and apixaban. TIhe results of the sensitivity analyses are shown in Supporting Information Figures S1-S3.
| Risk scores | C-statistics (95% CI) | P valuea | Risk threshold | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95% CI) |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| HAS-BLED | 0.65 (0.57-0.74) | 0.62 | Low risk | 38.2 (22.2-56.4) | 30.9 (27.9-33.9) | 93.2 (89.7-95.7) | 2.0 (1.1-3.4) |
| High risk | 61.8 (43.6-77.8) | 69.1 (66.1-72.1) | 98.0 (96.6-98.9) | 6.8 (4.3-10.3) | |||
| ORBIT | 0.64 (0.56-0.71) | Low risk | 29.4 (15.5-47.5) | 43.6 (40.4-46.8) | 94.4 (91.8-96.4) | 1.9 (0.9-3.4) | |
| Intermediate/high risk | 70.6 (52.5-84.9) | 56.4 (53.2-59.6) | 98.1 (96.6-99.1) | 5.6 (3.6-8.2) | |||
| Warfarin-naïve | |||||||
| HAS-BLED | 0.65 (0.51-0.79) | 0.88 | Low risk | 38.5 (13.9-68.4) | 31.3 (27.2-35.6) | 95.1 (90.5-97.8) | 1.5 (0.5-3.4) |
| High risk | 61.5 (31.6-86.1) | 68.7 (64.4-72.8) | 98.5 (96.6-99.5) | 4.9 (2.2-9.5) | |||
| ORBIT | 0.64 (0.51-0.77) | Low risk | 30.8 (9.1-61.4) | 41.1 (36.7-45.5) | 95.7 (92.1-98.0) | 1.4 (0.4-3.4) | |
| Intermediate/high risk | 69.2 (38.6-90.9) | 58.9 (54.5-63.3) | 98.6 (96.6-99.6) | 4.3 (2.0-7.9) | |||
| Warfarin-experienced | |||||||
| HAS-BLED | 0.66 (0.55-0.77) | 0.50 | Low risk | 38.1 (18.1-61.6) | 30.3 (26.1-34.9) | 91.0 (85.2-95.1) | 2.6 (1.1-5.0) |
| High risk | 61.9 (38.4-81.9) | 69.7 (65.1-73.9) | 97.4 (95.0-98.9) | 9.0 (4.9-14.8) | |||
| ORBIT | 0.63 (0.52-0.73) | Low risk | 28.6 (11.3-52.2) | 46.4 (41.7-51.2) | 93.1 (88.9-96.1) | 2.5 (0.9-5.4) | |
| Intermediate-high risk | 71.4 (47.8-88.7) | 53.6 (48.8-58.3) | 97.5 (94.6-99.1) | 6.9 (3.9-11.1) | |||
- CI, confidence interval; HAS-BLED = hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile inr, elderly, drug/alcohol usage;ORBIT = Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
- a P value for the comparison of C-statistics of bleeding scores.
4 DISCUSSION
When employing anticoagulation therapy, the goal is to maximize the benefit by preventing thrombosis and minimize the risk of adverse events, particularly bleeding.22 The ability to accurately predict those who are at risk for bleeding helps determine the net clinical benefit when balancing stroke prevention against the risk of bleeding, which is particularly relevant in Asian subjects.23 Several bleeding risk scores have been created to fulfil this function for NVAF patients.24 The risk of bleeding, however, results from a complex interplay among patients, drugs and health system factors. As a result, factors contributing to bleeding in one population may differ from those in another. All bleeding risk scores are developed from predominantly Caucasian populations living in developed countries.24 As a result, the types of factors identified from these cohorts along with the magnitudes of their contributions to the risk of bleeding may vary in different populations.
To address this knowledge gap, we evaluated the performance of the HAS-BLED and ORBIT scores in predicting major bleeding events in a cohort of Asian NVAF patients receiving long-term DOAC therapy. We selected these two scores since both scores have been validated in various populations, particularly among NVAF patients using DOACs. In addition, these two scores, particularly the HAS-BLED score, are the most commonly used scoring systems in contemporary trials and clinical practice.15, 24 The key findings from our study indicated that both bleeding risk scores had moderate predictive performance in our population. This finding is consistent with a previous study conducted by Mori et al in a Japanese NVAF population which showed modest utility of these scores.13 Despite some differences in design and size, the study population in our study shared a number of similarities with the study by Mori et al.13 The average age, body weight, renal function, common comorbidities and average HAS-BLED and ORBIT were almost identical. This may partly explain a similar finding between our study and that of Mori et al.13
A previous study suggested that patients who switched from warfarin to a DOAC may have a higher bleeding risk compared to warfarin-naïve patients.16 To address this issue, we evaluated the performance of the scores, not only on the overall cohort, but also on warfarin-naïve and warfarin-experienced groups. The results of our study showed that both scores showed moderate predictive performance on major bleeding regardless of prior warfarin exposure. We also performed sensitivity analysis based on type of DOAC. The results were largely similar to the main analysis, except in the case of apixaban in warfarin-naïve patients where the C-statistic was <0.5. This finding is most likely due to the very low number of events in this group leading to low statistical power.
Previously, the HAS-BLED score showed good performance in predicting the risk of major bleeding among Thai NVAF patients receiving warfarin therapy.25 That is in contrast with the finding from this study, which comprised all DOAC users. One potential explanation is the fact that the risk item “labile INR” may not be applicable to DOAC users. Labile INR or poor INR control has been shown to be a strong predictor of bleeding among warfarin users.26, 27 Previous studies set the score of “labile INR” to 0 when testing the predictive performance of the HAS-BLED score in DOAC users, which basically annuls the contribution of this important factor. We attempted to alleviate this issue by setting a score of 1 for patients receiving an inappropriately high dose of DOAC and a score of 0 for DOACs users receiving appropriate and lower doses. Despite this, the predictability of the HAS-BLED score remained modest.
At the same level of anticoagulation, Asian patients tend to suffer a higher rate of bleeding events than other racial groups.28-30 Several explanations for this have been theorized but definite reasons are still unknown.30 For vitamin K antagonists, variation in anticoagulation control appears to play an important role.26, 27 However, for DOACs, this is less clear. Whether the variation is partly a reflection of differences in some unmeasured baseline characteristics, the effect of the overall quality of care in Asian countries, genetic susceptibility to bleeding or other physiological determinants remains to be confirmed.29
Given the lack of a specific clinical risk score to predict bleeding in Asian populations, both HAS-BLED and ORBIT can still be used with moderate accuracy. One important benefit of applying a bleeding risk score in clinical practice is to draw attention to modifiable bleeding risk factors so that attempts can be made to alleviate or eliminate the risk, or to perform frequent follow-up for those at high risk of bleeding.31, 32 This is because the risk of bleeding is dynamic and can constantly change over time.33 A recent controlled trial showed that, by using the HAS-BLED score, interventions aiming to reduce or alleviate modifiable bleeding risk factors led to a lower rate of major bleeding events at 1 year compared to the usual care.34 For this reason, the HAS-BLED score may have the advantage over ORBIT based on its simplicity and presence of modifiable bleeding risk factors while ORBIT is largely based on nonmodifiable criteria.
Our study has some limitations. First, the study sites were all tertiary-care hospitals due to the availability of DOACs at the time of the study, which was limited to large hospitals. Generalizability to secondary hospitals and among patient populations living in rural areas may be limited. Second, it is possible that some events were not captured since the study design was retrospective. However, we believe that this possibility would be low since major bleeding was a catastrophic event and almost always led to hospitalization. Third, we followed up the case from the time of initiation of the first DOAC until the first occurrence of event or end of the study period. Any subsequent changes in type of DOAC that occurred later were not captured since we wanted to avoid duplicate patients. As a result, repeated bleedings if occurred in a patient but with other DOACs would not be captured. In addition, since we calculated the risk scores at the time of treatment initiation, the effect of dynamic changes in the risk category of the patients during the follow-up may potentially affect our findings. Fourth, we performed this study in a Thai population. Therefore, there is a possibility that these risk scores may perform differently among other Asian groups such as Han Chinese and South Indians. Further studies from various Asian groups are needed. Lastly, the number of bleeding events in our study was relatively small compared to large national registries. As a result, larger trials with longer follow-up and more events should be performed to confirm our findings. However, the findings from our study corroborate the findings from the previous study conducted in a Japanese population.
5 CONCLUSION
The HAS-BLED and the ORBIT bleeding risk prediction scores have similarly moderate predictive performances in an Asian NVAF population receiving DOACs. Despite the moderate performance, the risk score is still useful to clinicians caring for the Asian population, a population with higher bleeding rate than other racial groups. Frequent assessment of bleeding risk, mitigation or elimination of modifiable bleeding risk factors and the employment of regular and frequent follow-up for those at high risk of bleeding should be implemented.
ACKNOWLEDGEMENTS
All authors have made substantial contributions to the study. P.W., K.K., W.W., A.P. and S.N. were involved in the conception and design of the study. P.W., W.W., A.P. and S.N. were responsible for the acquisition of data. P.W., K.K., G.L. and S.N. were responsible for data analysis and contributed to data interpretation. P.W., S.N. and G.L. were responsible for drafting the manuscript. All authors approved the final manuscript. We would like to thank the University Hospital Network, Thailand (UHOSNET) for their contribution in a previous study by Wattanaruengchai et al17 which made this present study possible.
COMPETING INTERESTS
G.Y.H.L. serves as consultant and speaker from BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees were received personally. All other authors have no actual or potential conflict of interest capable of influencing judgment on the part of any author of this work.
AUTHOR CONTRIBUTORS
All authors have made substantial contributions to the study. PW, SN, WW and AP involved in the conception and design of the study. PW, WW and AP were responsible for acquisition of data. PW, SN, KK and GL were responsible for data analysis. PW, SN, KK and GL contributed in the data interpretation. PW, SN and GL were responsible for drafting of manuscript. All authors approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author and with the permission of the participating hospitals.